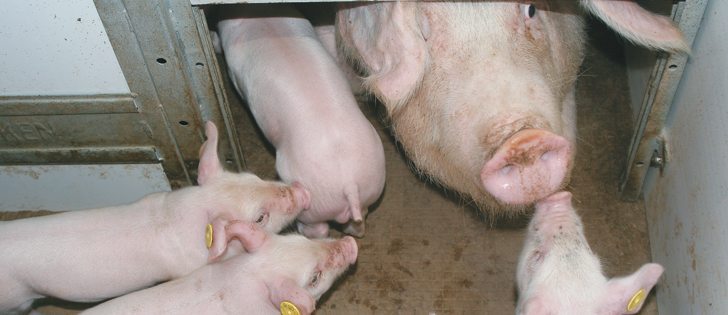
CHICAGO (Reuters) — Zoetis Inc. has received a conditional licence from the U.S. Department of Agriculture for its vaccine against a deadly piglet virus and will begin selling it this month in the United States, the company said last week.
With its new vaccine, Zoetis, the world’s largest animal-health company, joins a growing push by both the agriculture and pharmaceutical industries to combat the spread of porcine epidemic diarrhea virus, which has killed about 13 percent of the U.S. hog herd over the past year.
Results from preliminary studies on the product have been “promising,” said Joelle Hayden, spokesperson for USDA’s Animal and Plant Health Inspection Service.
Read Also

The Western Producer Livestock Report – November 13, 2025
Western Producer Livestock Report for November 13, 2025. See U.S. & Canadian hog prices, Canadian bison & lamb market data and sales insights.
“They’ve shown sufficient data that we think the vaccine will be effective,” she said about Zoetis.
The vaccine comes as veterinarians warn that outbreaks of the virus are expected to surge this fall and winter because PED thrives in cold weather.
Zoetis’ product means that hog farmers now have two PED vaccine options. Earlier this year, USDA granted a similar conditional approval to Iowa-based Harris vaccines for its PED vaccine.
Merck & Co. Inc.’s animal health unit is also working on a PED vaccine. Zoetis was spun off from drugmaker Pfizer Inc. last year.
The fast-moving virus has killed an estimated eight million piglets in the U.S. since it was first identified last year, pushing pork prices to record highs.
The conditional licence will allow Zoetis to sell the two-dose inactivated vaccine directly to veterinarians and hog farmers alike for use on healthy pregnant sows, while the company continues to conduct further tests both in research laboratories and in field tests at customers’ farms.
Zoetis declined to comment on the company’s research, how successful the vaccine has been in reducing mortality rates in baby pigs or what field tests have shown so far. Company officials did not say how much the vaccine will cost.
“We have proven at least some efficacy of those antibodies produced with the sow of being transferred to the baby piglets,” Gloria Basse, vice-president of the company’s U.S. pork marketing, said in an interview.
Zoetis said it was exploring new international markets, including Canada, Mexico and Japan, for the vaccine.
Concerns over the virus have fuelled contamination fears among U.S. trading partners and prompted a four-month ban on imports of live U.S. pigs into China.
“Really, anywhere there’s a customer need, that’s where we are going to be involved in the discussion around product relevance,” said Catherine Knupp, president of research and development for Zoetis.
Knupp said the company would look for local partnerships for such projects.