This is the fourth column on macro-nutrients. This is part one of a two-part column on nitrogen.
Nitrogen is by far the most important, the most complicated, the most controversial and possibly the least understood nutrient commonly used by farmers.
Nitrogen is critical to making crops grow, mature and produce grain. A crop lacking nitrogen produces small, pale green plants, yields poorly, has poor quality grain and is prone to diseases. On the other hand, crops with adequate nitrogen, look vigorous and healthy. The dark green colour we equate to a healthy plant is a result of adequate nitrogen.
Read Also

Volatile temperatures expected for this winter
DTN is forecasting a lot of temperature variability in the Canadian Prairies this winter. Precipitation should be close to average.
With more than two million tonnes used in Western Canada in 2018, nitrogen accounts for more tonnes of fertilizer than all the other nutrients combined. It also accounts for more yield increase than all other nutrients combined.
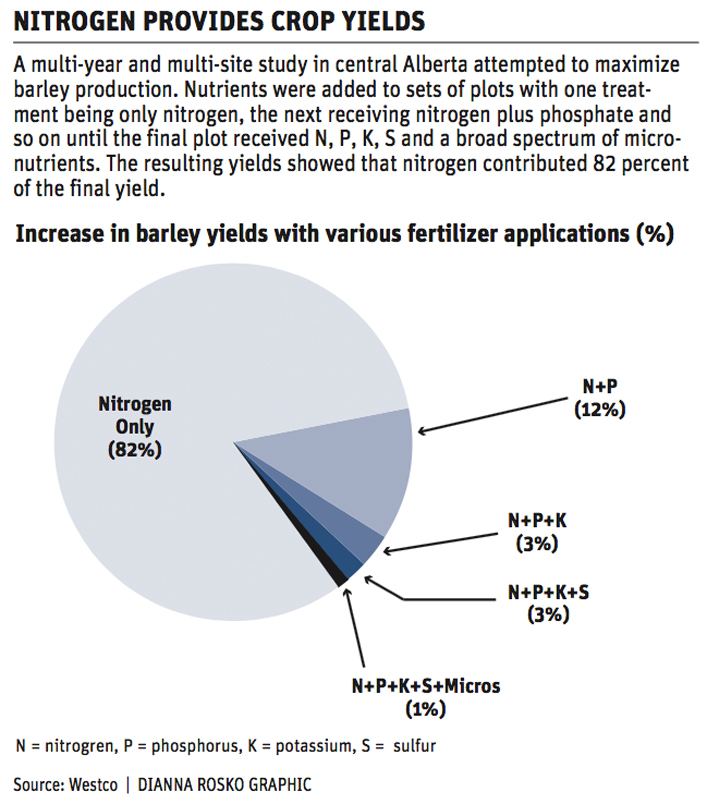
An interesting set of experiments were put out by Westco Fertilizer in the late 1990s. This was a multi-year and multi-site study in central Alberta that tried to maximize barley production.
Nutrients were added to sets of plots with one treatment being only nitrogen, the next receiving nitrogen plus phosphate and so on until the final plot received nitrogen, phosphorus, potassium and sulfur and a broad spectrum micro-nutrient application.
All were applied at levels that were significantly higher than soil tests recommended but not to toxic levels.
The study revealed that, on average, nitrogen contributed to 82 percent of the final yield, followed by phosphate at 12 percent, potassium at three percent, sulphur at two percent and finally the micro-nutrient mix at one percent. While remembering that this was a barley study in a limited geographic area, I believe that these numbers could be used today for most non-manured fields in Western Canada, with nitrogen delivering 75 to 85 percent of the yield punch.
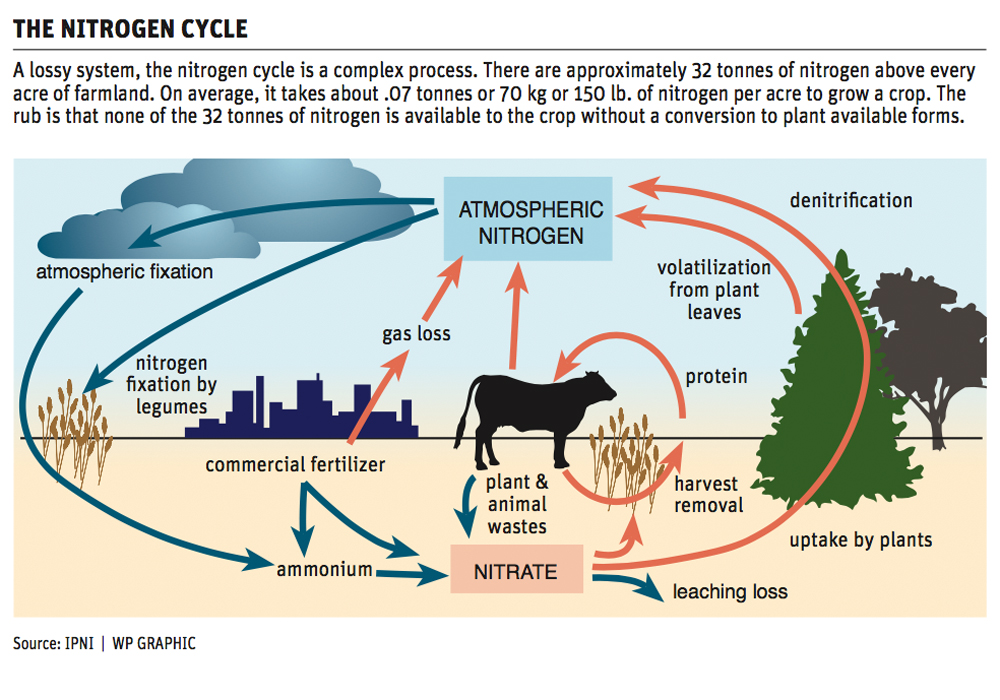
Nitrogen’s system is much different than other nutrients because it has many internal cycles and is leaky due to a number of loss-mechanisms built into it. Unlike other nutrients, nitrogen also has a number of addition points. It can enter from the atmosphere, from the soil or from the addition of fertilizer nitrogen. And when it comes to fertilizers, nitrogen can be found, not only in different products but also in different forms, each behaving differently. This makes nitrogen the key element when talking about fertilizers and also the most complex nutrient in how it is used.
To understand how nitrogen works in your fields, you must understand the nitrogen cycle.
There are about 32 tonnes, 70,000 pounds, of nitrogen above every acre of farmland in Canada or the United States or anywhere in the world. On average, it takes about .07 tonnes or 70 kilograms or 150 lb. of nitrogen per acre to grow a crop. The rub is that none of the 32 tonnes of nitrogen in the air is available to the crop without a conversion to plant-available forms.
There are three ways to convert atmospheric nitrogen (N2) to plant-available nitrogen (NH4+ or NO2-). The first is through atmospheric fixation. The enormous energy of lightning breaks nitrogen molecules and enables their atoms to combine with oxygen in the air forming nitrogen oxides. These dissolve in rain, forming nitrates or nitric acid, that are carried to the earth. Atmospheric nitrogen fixation contributes five to eight percent of the total nitrogen fixed. It may account for five to 20 lb. per acre annually.
The second process that converts atmospheric nitrogen to plant available nitrogen is biological fixation (BF). Bacteria are responsible for this process. Bacteria in terrestrial and aquatic environments participate in this process. These organisms must have a special enzyme known as dinitrogenase to be able to do this.
These bacteria reduce atmospheric nitrogen to ammonia, which can be used to make other biological compounds.
Biological nitrogen fixation requires energy. Symbiotic nitrogen-fixing micro-organisms are those nitrogen fixing bacteria that live in close proximity to plant roots and can obtain energy materials from the plants. The symbiotic relationship between bacteria called rhizobia and legumes, for example, clover and peas, can provide large amounts of nitrogen to the plant and can have a significant impact on agriculture and the environment.
The symbiosis between legumes and the nitrogen-fixing rhizobia occurs within bumps or nodules on the roots. The plant supplies energy materials to the bacteria, which in turn reduces atmospheric nitrogen to ammonia. This ammonia is transferred from the bacteria to the plant to meet the plant’s nutritional nitrogen requirements for the synthesis of proteins, enzymes, nucleic acids, chlorophyll, and so forth. In many cases, the entire nitrogen requirements of crops such as peas or faba beans are provided by this relationship. This can be as high as 150 lb. of nitrogen per acre.
The third way nitrogen is taken from atmospheric nitrogen is industrial fixation. In the latter decades of the 19th century and early in the 20th century, a few processes were discovered that converted atmospheric nitrogen into nitrogen compounds.
By far the most commonly used method is called the Haber–Bosch process. It was named the greatest innovation of the 20th century and uses nitrogen from the air, hydrogen and high pressure to convert the nitrogen to ammonia on large industrial scales. This is the process used by modern nitrogen fertilizer plants such as those found across Western Canada at Brandon, Man., Belle Plaine, Sask., and in Alberta at Medicine Hat, Joffre, Carseland and Fort Saskatchewan.
With the exception of the Joffre plant, they all use hydrogen from natural gas and nitrogen from the air to produce ammonia. The Joffre plant uses waste hydrogen from adjacent industrial operations. In many cases, the ammonia is further processed into products such as urea, ammonium sulphate or ammonium nitrate.
As indicated above, the nitrogen cycle is leaky. There are several ways that nitrogen can be lost from the system. These include volatilization, leaching and denitrification.
All ammonium and ammonia-based fertilizers, including manure, have the potential for ammonia volatilization — the loss of nitrogen to the air as ammonia gas.
One product that has a great potential for volatilization losses is surface-applied urea. Studies done at University of Manitoba showed losses of 38 to 46 percent of urea nitrogen during five days at 25 C versus less than seven percent loss when temperatures are 15 C.
Other studies under no tillage conditions a found 40 percent and 88 percent loss of urea nitrogen after seven days in May and July.
Leaching occurs when nitrate is dissolved in soil water and moves with the water. This process can move the nitrate out of the rooting zone or even into surface or groundwater. There, it can act as a pollutant and can damage the environment. Nitrate leaching usually occurs on coarse textured sandy soils after a heavy rainfall or irrigation. Leaching losses can also be high on tile-drained fields after heavy rainfall where high levels of nitrate nitrogen are present.
Nitrate nitrogen is also at risk of being lost through denitrification. Denitrification is a biological process where certain classes of microbes use nitrate as a food source. Under anaerobic, flooded conditions, these microbes rob oxygen from the nitrate to survive. Through a number of steps, the nitrate is converted to nitrite, nitrous oxide and finally nitrogen gas. Nitrous oxide is a significant greenhouse gas, rated as being 300 times more potent than carbon dioxide.
One final loss mechanism is immobilization. This is another biological process in which microbes take nitrogen from the soil to enable them to break down or decay crop residues. For example, the presence of a high amount of cereal straw or corn stalks in the soil will result in immobilization processes taking place. That is because these materials have a much higher carbon content than nitrogen.
Immobilization results in plant-usable forms of nitrogen in the soil becoming unavailable for the crop’s growth. This nitrogen is used by micro-organisms in the decomposition process of cereal straw or corn stalks. Once the cereal straw or corn stalks have become highly decayed, immobilization stops and mineralization starts. So, in reality, this is not a loss mechanism but really a delay mechanism. That is, plant-usable forms of nitrogen, such as ammonium, become available again.
Immobilization processes generally do not take place when a legume crop, such as peas or soybeans, are grown because they have a lower amount of carbon as compared to nitrogen. Mineralization will likely be the dominant process when these stubble residues are found and when they are incorporated into the soil using tillage.
Keep posted for my next column where I discuss nitrogen and Liebig’s law of the minimum, the law of diminishing returns and the 4 Rs of nitrogen stewardship.