This is the second of a four-part series on advanced efficiency fertilizer. Links to other stories in this series are below.
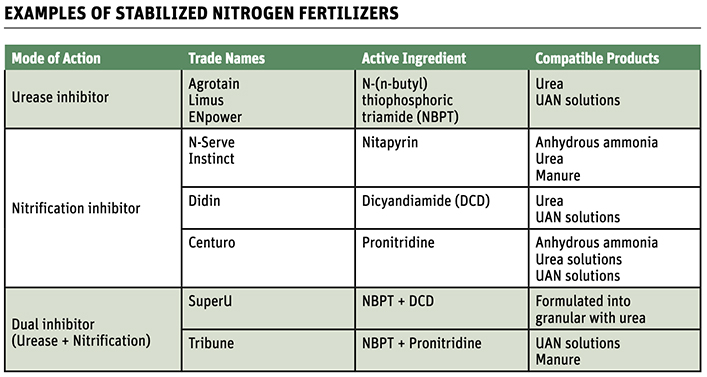
The enhanced efficiency fertilizers (EEF) available to farmers in Canada include urease inhibitors, nitrification inhibitors, dual inhibitors (both urease and nitrification) and polymer coated urea, or slow-release products.
Urease inhibitors
Read Also

Short rapeseed crop may put China in a bind
Industry thinks China’s rapeseed crop is way smaller than the official government estimate. The country’s canola imports will also be down, so there will be a lot of unmet demand.
Urea is the most popular source of nitrogen fertilizer in Canada. When applied to the soil it reacts with water and the soil enzyme urease, and then is converted to ammonium.
Related stories in this series:
- Part 1 – Enhanced efficiency adoption is low
- Part 3 – When do EEFs make agronomic sense?
- Part 4 – Can you cut fertilizer rates with EEFs?
The chemical reaction is shown in Figure 1:
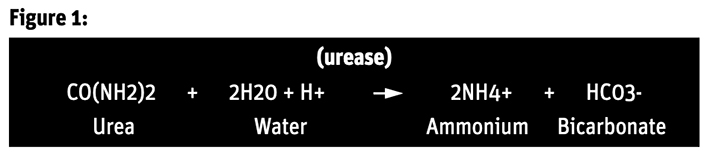
“This conversion… is called urea hydrolysis,” says a Kansas State University agronomy website. “In this reaction hydrogen ions (H+) are consumed, causing the soil pH near the fertilizer to rise. If the pH rises above seven, a significant amount of gaseous ammonia can form in soil for a few days following urea application.”
Fine soils that have more organic matter and low pH, will likely produce less ammonia.
“At the other extreme, soils that are sandy and low in organic matter, especially those with a high pH, allow more ammonia to be formed from urea hydrolysis,” K-State says.
Temperature and moisture also affect urea hydrolysis, as cool conditions will slow the chemical reaction.
Urease inhibitors, sold under brand names such as Agrotain, interfere with the process and reduce the amount of NH3 released to the atmosphere, particularly with surface applications.
“When the urease enzyme approaches the urea fertilizer, it binds with the inhibitor and causes the enzyme to be non-functional. So, basically it keeps the active enzymes from attacking the urea granules to produce ammonia,” Mario Tenuta, a University of Manitoba soil scientist, told the Manitoba Co-operator in 2021.
Craig Drury, an Agriculture Canada research scientist in Harrow, Ontario, has conducted experiments looking at the benefits of EEFs such as urease inhibitors.
Data from Ontario shows that N leakage to the atmosphere can be significant.
“In a study I published in 2017… ammonium loss for those years amounted to 50 per cent of the applied N. So that’s huge,” Drury said, referring to research done in southwestern Ontario, where urea was broadcast on the soil surface with no inhibitors.
“That doesn’t occur every year. (But) if you look at the magnitude of loss from a crop standpoint, ammonium loss can have an… impact on the total amount of nitrogen available to the plant.”
If the fertilizer is treated with a urease inhibitor, it gives the urea more time to solubilize and become available to the crop. A meta-analysis of 182 studies on EEFs, published in 2022, found that urease inhibitors can reduce volatilization losses by 50 per cent.
Nitrification inhibitors
When urea is transformed into ammonium (NH4+), it’s in a form that’s available to the plant.
The term “plant-available nitrogen” refers to forms of nitrogen that plants most readily absorb, mostly ammonium and nitrate.
“Soil nitrogen exists in three general forms: organic nitrogen compounds, ammonium (NH₄⁺) ions and nitrate (NO₃⁻) ions,” says cropnutrition.com, a website for Mosaic, a fertilizer company.
“The majority of plant-available nitrogen is in the inorganic forms NH₄⁺ and NO₃⁻ (sometimes called mineral nitrogen). Ammonium ions bind to the soil’s negatively charged cation exchange complex…. Nitrate ions do not bind to the soil solids because they carry negative charges, but exist dissolved in the soil water, or precipitated as soluble salts under dry conditions.”
However, the ammonium can be quickly converted to nitrate in a process called nitrification.
Soil bacteria, Nitrosomonas, convert NH4+ to nitrite (NO2-), then nitrobacter, another bacteria, converts the nitrite to nitrate (NO3-).
When nitrogen is in the nitrate form, the two primary losses are leaching and denitrification.
“Leaching occurs when excess water causes a downward movement of nitrate-nitrogen through the soil profile,” says fieldcropnews.com, an Ontario ministry of agriculture, food and rural affairs publication.
“Sandy soils are more prone to leaching loss than loamy or clay soils, as water moves more rapidly through the soil profile,”
Denitrification is the conversion of nitrate to gaseous forms like nitrogen gas (N2) and nitrous oxide (N2O).
“During denitrification, bacteria that cannot get adequate oxygen from soil air will instead scavenge oxygen from nitrate, releasing some as nitrogen gas and some as N2O,” says an On-Farm Climate Action Fund fact sheet, published by Agriculture Canada.
The scale of loss depends upon soil type, soil moisture and temperature.
It’s a bigger risk in clay and loamy soils when the soil is saturated.
Nitrification inhibitors (NI) contain organic compounds like nitrapyrin, which
disrupt the soil microbes.
“Nitrification inhibitors block this process, either by inhibiting the growth of nitrifying bacteria or blocking the enzyme which aids the process,” says the On-Farm Climate Action Fund document.
So, more nitrogen remains as NH4+, reducing the concentration of nitrate NO3- in the soil.
That’s critical for cutting N2O losses during times of the year when the soil is saturated, like the spring.
Research shows that nitrification inhibitors can significantly reduce the amount nitrous oxide emissions from soil.
The meta-analysis of 182 studies on EEFs, found that nitrification inhibitors cut N2O emissions by 49 per cent, on average.
Dual inhibitors and controlled release products
Another option for growers is a dual inhibitor that includes a nitrification inhibitor and a urease inhibitor.
The primary dual inhibitors in the Canadian market are SuperU and Tribune.
Because they reduce losses of both ammonia and nitrous oxide, by combining the actions of both inhibitors, the benefits of a dual inhibitor can be more significant than a single inhibitor.
For slow-release products, the most common polymer-coated urea is ESN.
The polymer coating allows water to seep into the granule and dissolve the urea inside.
“Then the dissolved urea slowly diffuses through the coating depending on the temperature and moisture in the soil,” says the On-Farm Climate Action Fund fact sheet. “The delay in nitrogen release is from two-six weeks, depending on product and conditions.”
Ammonia losses more important for yield than nitrous oxide
Preventing nitrous oxide emissions from fertilizer is a policy priority in Canada and around the globe because N2O is a powerful greenhouse gas. One kg of N2O released to the atmosphere is equal to about 300 kg of carbon dioxide.
However, nitrogen leakage as N2O is small compared to the NH3 released through volatilization.
“The potential loss in the form of ammonia, or the potential loss in the form of nitrate by leaching, those losses as a percentage of the nitrogen applied are much higher,” Bruulsema said.
“If you have urea and you broadcast it on the surface and… no incorporation, you can expect losses of 20 to 50 per cent of the nitrogen.”
If the nitrogen fertilizer is applied to a sandy soil in a year with excess moisture, “you can expect to lose 20 to 30 per cent by nitrate leaching over the course of the growing season,” Bruulsema added.
In 2020, Drury and his team published a paper on ammonia volatilization and N2O emissions in the Soil Science Society of America Journal. The study was done at the Eugene Whelan Experimental Farm in Woodslee, Ont.
They found:
- With broadcast and incorporated urea (no inhibitors), NH3 losses were 14.1 kg/Ha (average of three years)
- N2O losses were 1.12 kg/Ha.
- So, NH3 losses were nearly 13 times higher than nitrous oxide
- With broadcast urea (no inhibitors), NH3 losses were 21.3 kg/Ha and N2O losses were 2.13 kg/Ha.
- Ammonia losses were 10 times higher than N2O
The results from Ontario and data from similar studies indicate that NH3 leakage may have a direct impact on crop yields.
“If you’re trying to control all the losses that could be economical to you as a farmer, you would have much more focus on reducing the ammonia emissions or the nitrate loss from (leaching),” Bruulsema said. “As a side benefit, (EEFs) reduce nitrous oxide emissions. But that’s only small loss.”
















